The anisotropic behaviour of liquid crystals is caused by the elongated shape of the molecules. The physical properties of the molecules are different when measured parallel or perpendicular to their length, and residual alignment of the rods in the fluid leads to anisotropic bulk properties. This residual alignment occurs as a result of preferential packing arrangements, and also electrostatic interactions between molecules that are most favourable (lowest in energy) in aligned configurations.
There are three types of liquid crystal: nematic, smectic and cholesteric. In the liquid crystalline phase, the vector about which the molecules are preferentially oriented, n, is known as the "director". The long axes of the molecules will tend to align in this direction.
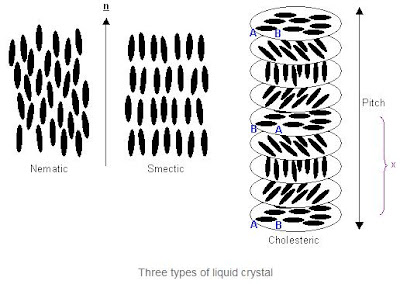
In addition to the long range orientational order of nematic liquid crystals, smectic liquid crystals also have one dimensional long range positional order, the molecules being arranged into layers.
A cholesteric (or twisted nematic) liquid crystal is chiral: the molecules have left or right handedness. When the molecules align in layers, this causes the director orientation to rotate slightly between the layers, eventually bringing the molecules back into the original orientation. The distance required to achieve this is known as the pitch of the twisted nematic, as seen in the diagram above. The pitch is not equal to the distance marked x, because only 180º of rotation occurs over this length, so the molecules are aligned antiparallel to their starting orientation.
When viewed between crossed polars, thin films (approximately 10mm thick) of liquid crystals exhibit schlieren textures, as seen in the micrograph below, which shows a nematic liquid crystalline polymer.
 Micrograph of nematic liquid crystalline polymer, courtesy of Professor TW Clyne and the DoITPoMS Micrograph Library (click on image to view larger version, or view full library record for the micrograph)
Micrograph of nematic liquid crystalline polymer, courtesy of Professor TW Clyne and the DoITPoMS Micrograph Library (click on image to view larger version, or view full library record for the micrograph) The black brushes are regions where the director is either parallel or perpendicular to the plane of polarisation of the incident radiation, and the points at which the brushes meet are known as disclinations.
If the temperature of a liquid crystal is raised, the constituent molecules have more energy, and are able to move and rotate more, so the liquid crystal becomes less ordered. As a result, the magnitude of the anisotropy of the bulk properties of the liquid crystal decreases, usually eventually resulting in an isotropic fluid.
Liquid crystals are used in many different applications, for example the displays on calculators, digital watches and mobile phones.
ไม่มีความคิดเห็น:
แสดงความคิดเห็น